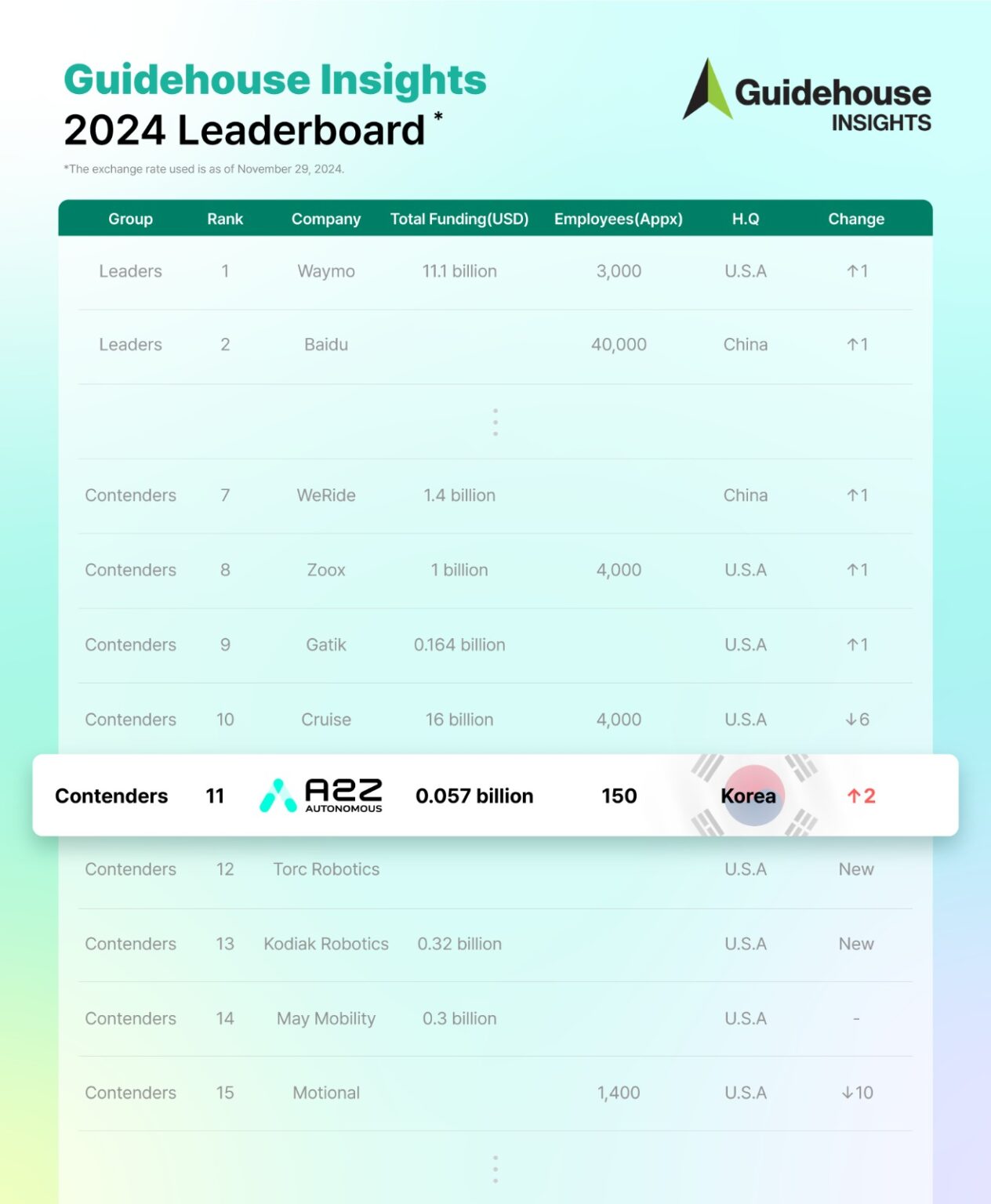
AUTONOMOUS A2Z has risen to 11th place in Guidehouse Insights’ 2024 Automated Driving Leaderboard, moving up two spots from last year’s 13th-place debut and marking its second consecutive year in the rankings, further reinforcing its growing influence in the autonomous vehicle (AV) sector.
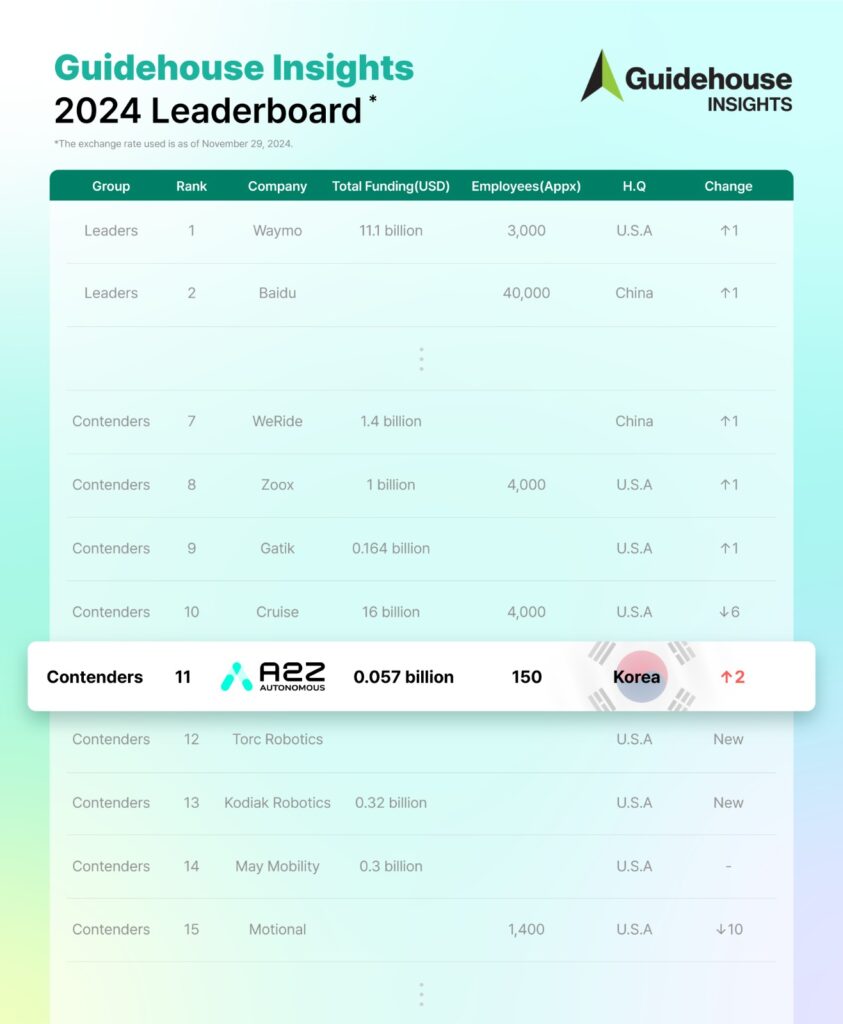
Guidehouse Insights, a U.S.-based market research firm, evaluates AV companies annually based on corporate vision, market strategy, partnerships, product development, and technological capabilities. This year, the ranking expanded from 16 to 20 companies, reflecting intensifying global competition as autonomous trucking firms entered the list.
As the only South Korean company included, AUTONOMOUS A2Z continues to lead the country’s AV ecosystem. The firm also advanced from the “Challengers” category to “Contenders,” signaling progress in commercialization and market positioning.
Commercialization and Global Expansion Drive Growth
Guidehouse Insights attributed AUTONOMOUS A2Z’s improved ranking to its forward-thinking strategy that focuses on autonomous shuttle, international partnerships and government-supported commercialization efforts. The company received strong scores for corporate vision (85 points) and market entry strategy (75 points), reflecting its forward-thinking approach.
A2Z plans to launch its ROii Level 4 autonomous shuttle in late 2025. The company is accelerating global expansion. Unlike competitors prioritizing robotaxis, AUTONOMOUS A2Z is focusing on autonomous shuttles for public transportation, a strategy gaining industry traction.
To further strengthen its international presence, the firm has established two strategic joint ventures:
– A2D (Abu Dhabi Autonomous Driving) with Abu Dhabi listed AI firm to form UAE-South Korea Joint Venture for Autonomous Mobility to expand in the Middle East
– A2G (Autonomous2Global) with KILSA Global, a Singapore-based market entry services provider, to accelerate expansion into Southeast Asia.
Additionally, AUTONOMOUS A2Z has achieved 96% localization in AV manufacturing through partnerships with Samsung SDI and leading Korean auto parts suppliers, contributing to its strong partnership score (72 points) in the Guidehouse Insights evaluation.
Industry Giants Maintain Lead, But South Korea Gains Ground
Despite the rise of new challengers, U.S. and Chinese companies continue to dominate the AV industry, with 15 American and three Chinese firms securing spots in the rankings. Waymo remains at No. 1, followed by Baidu at No. 2 and Mobileye at No. 3.
Among the “Contenders,” Nvidia ranks No. 4, while Motional, a Hyundai-Aptiv joint venture, holds 15th place. Tesla, despite its aggressive push in autonomous driving, ranks last at No. 20.
AUTONOMOUS A2Z operates with the lowest cumulative funding among the top 20 companies, at KRW 52 billion ($39 million) as of December 2023, yet continues to expand steadily.
Government Backing Fuels AUTONOMOUS A2Z’s Climb in Global AV Rankings
AUTONOMOUS A2Z’s ascent in the latest Guidehouse Insights rankings underscores its ability to compete on the global stage despite operating with significantly lower funding than industry heavyweights. CEO Ji-hyeong Han credits the company’s progress to South Korea’s forward-leaning regulatory framework and strong government support for AV commercialization.
“South Korea’s proactive policies, such as the Level 4 AV sales framework, have been instrumental in accelerating commercialization by allowing legally certified autonomous vehicles to enter the market,” Han said. “The K-City testbed, provided by the Korea Transportation Safety Authority (KATRI), has also played a critical role in advancing our technology. We’re deeply appreciative of this support.”
Looking ahead, Han reaffirmed the company’s commitment to innovation and global expansion, positioning South Korea as a key player in the evolving autonomous mobility landscape.
“We will continue to push the boundaries of AV technology and scale our market presence, ensuring South Korea plays a leading role in shaping the future of transportation.”
Scaling a Distinctive Strategy for Global Growth
Founded in 2018 by former Hyundai Motor engineers (Ji-hyeong Han, Young-chul Oh, Byung-yong You, and Myung-seon Heo) AUTONOMOUS A2Z has quickly emerged as South Korea’s premier AV startup. The company operates the country’s largest autonomous vehicle fleet (51 units) and has logged 570,000 km of real-world autonomous driving experience.
While many competitors are doubling down on robotaxis, AUTONOMOUS A2Z has carved out a niche in autonomous shuttles for public transit, a market gaining traction as cities rethink urban mobility. The company’s roadmap is closely aligned with South Korea’s national mobility strategy, which aims to commercialize Level 4 AVs by 2027.
With an ambitious expansion push into the Middle East and Southeast Asia, AUTONOMOUS A2Z is cementing its foothold in the global autonomous transportation sector—proving that strategic execution, not just capital, drives industry leadership.